What does reducing agent mean?
Definitions for reducing agent
re·duc·ing agent
This dictionary definitions page includes all the possible meanings, example usage and translations of the word reducing agent.
Princeton's WordNet
reducing agent, reducer, reductantnoun
a substance capable of bringing about the reduction of another substance as it itself is oxidized; used in photography to lessen the density of a negative or print by oxidizing some of the loose silver
GCIDE
Reducing agentnoun
a substance that causes reduction of another substance in a chemical reaction, as by donating electrons or adding hydrogen atoms; as, lithium hydride is a powerful reducing agent.
Wiktionary
reducing agentnoun
Any substance that reduces, or donates electrons to, another; in so doing, it becomes oxidized.
Wikipedia
Reducing agent
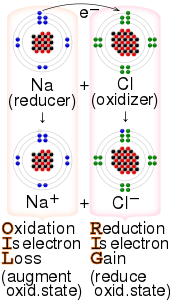
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an electron recipient (called the oxidizing agent, oxidant, oxidizer, or electron acceptor). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/donates electrons", that "is oxidized", and that "reduces" is called the reducer or reducing agent, while the agent whose oxidation state decreases, that "gains/accepts/receives electrons", that "is reduced", and that "oxidizes" is called the oxidizer or oxidizing agent. For example, consider the overall reaction for aerobic cellular respiration:
ChatGPT
reducing agent
A reducing agent, in chemistry, refers to a substance that causes a decrease in the oxidation state of another substance by donating electrons during a redox reaction, resulting in the reduction of the latter substance. The reducing agent itself gets oxidized in the process, as it loses electrons.
Wikidata
Reducing agent
A reducing agent is the element or compound in an oxidation-reduction reaction that donates an electron to another species. Because the reducing agent is losing electrons, we say it has been oxidized. This means that there must be an "oxidizer"; because if any chemical is an electron donor, another must be an electron recipient. Thus reducers are "oxidized" by oxidizers and oxidizers are "reduced" by reducers; reducers are by themselves reduced and oxidizers are by themselves oxidized. For example, consider the following reaction:4−3−− The reducing agent in this reaction is ferrocyanide. It donates an electron, becoming oxidized to ferricyanide. Simultaneously, the oxidizer chlorine is reduced to chloride. In organic chemistry, reduction more specifically refers to the addition of hydrogen to a molecule, though the aforementioned definition still applies. For example, benzene is reduced to cyclohexane in the presence of a platinum catalyst: In organic chemistry, good reducing agents are reagents that deliver H2.
Matched Categories
Numerology
Chaldean Numerology
The numerical value of reducing agent in Chaldean Numerology is: 2
Pythagorean Numerology
The numerical value of reducing agent in Pythagorean Numerology is: 2
Translations for reducing agent
From our Multilingual Translation Dictionary
- pelkistinFinnish
- bahan pereduksiIndonesian
- 還元剤Japanese
- indirge maddeTurkish
Get even more translations for reducing agent »
Translation
Find a translation for the reducing agent definition in other languages:
Select another language:
- - Select -
- 简体中文 (Chinese - Simplified)
- 繁體中文 (Chinese - Traditional)
- Español (Spanish)
- Esperanto (Esperanto)
- 日本語 (Japanese)
- Português (Portuguese)
- Deutsch (German)
- العربية (Arabic)
- Français (French)
- Русский (Russian)
- ಕನ್ನಡ (Kannada)
- 한국어 (Korean)
- עברית (Hebrew)
- Gaeilge (Irish)
- Українська (Ukrainian)
- اردو (Urdu)
- Magyar (Hungarian)
- मानक हिन्दी (Hindi)
- Indonesia (Indonesian)
- Italiano (Italian)
- தமிழ் (Tamil)
- Türkçe (Turkish)
- తెలుగు (Telugu)
- ภาษาไทย (Thai)
- Tiếng Việt (Vietnamese)
- Čeština (Czech)
- Polski (Polish)
- Bahasa Indonesia (Indonesian)
- Românește (Romanian)
- Nederlands (Dutch)
- Ελληνικά (Greek)
- Latinum (Latin)
- Svenska (Swedish)
- Dansk (Danish)
- Suomi (Finnish)
- فارسی (Persian)
- ייִדיש (Yiddish)
- հայերեն (Armenian)
- Norsk (Norwegian)
- English (English)
Word of the Day
Would you like us to send you a FREE new word definition delivered to your inbox daily?
Citation
Use the citation below to add this definition to your bibliography:
Style:MLAChicagoAPA
"reducing agent." Definitions.net. STANDS4 LLC, 2025. Web. 6 Mar. 2025. <https://www.definitions.net/definition/reducing+agent>.






Discuss these reducing agent definitions with the community:
Report Comment
We're doing our best to make sure our content is useful, accurate and safe.
If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we'll take care of it shortly.
Attachment
You need to be logged in to favorite.
Log In